Glyburide, an oral medication prescribed to treat type II diabetes, may one day save soldiers from dying of secondary, blast-related brain injuries sustained in armed combat. Researchers at Washington University School of Medicine are currently testing this potential new use of the drug.
And if the medication works prophylactically as researchers hope, a daily dose might also help high-impact athletes, like football players, avoid similar life-threatening injuries.
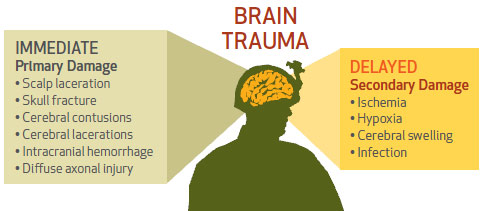
Traditionally used as an antiglycemic medication, glyburide is known to block access to the brain’s sulphonylurea (SUR1) receptors, thereby potentially preventing brain edema, swelling and intracranial pressure caused by secondary brain injury— effects that can occur in the days and weeks following the initial injury. In combat, these injuries often result from invisible blast waves produced by improvised explosive devices.
Research in animals has shown that glyburide works in preventing the harmful effects of secondary brain injury, but works better if it’s taken before the injury occurs.
And that’s where the local, phase 1 study comes into play.
“The first phase of the trial will be a proof of concept,” says Grant Bochicchio, MD, MPH, Edison Professor and chief of acute and critical care surgery, and principal investigator of the Glyburide Healthy Volunteer Study. The study’s goal is to determine the safety and tolerability of low-dose glyburide in healthy young adults. Specifically, the trial seeks to support a hypothesis that 1.5 mg/dl daily of the drug is safe both cognitively and physically. The U.S. Department of Defense is sponsoring the study.
“The dose is so low that we don’t anticipate any issues, but you never know until you do it,” says Bochicchio, who also oversees trauma services at Barnes-Jewish Hospital.
The week-long trial will involve 21 healthy subjects (both men and women) between 18 and 40 years of age who have no medical illnesses, no co-morbidities—and no diabetes. Subjects will be randomized to either oral glyburide or a placebo.
Volunteers will become inpatients at the medical center’s Clinical Research Unit within the Center for Applied Research Sciences. They will receive food that replicates the military’s Meals Ready To Eat and run on a treadmill three times a day to simulate the same sort of strenuous activity and caloric burn experienced on a battlefield.
Participants will be closely monitored to see if they become hypoglycemic while on the treadmill or suffer any ill effects from hypoglycemia. “We will know in real time if there are any adverse results,” says Bochicchio. “It will be very obvious.”
The study also will include cognitive tests. “We want to make sure participants’ cognitive and dexterity performance is not inhibited by the drug,” Bochicchio says. Therefore, participants will perform computerized tests before and after the trial to gauge their memory and their understanding of spatial relationships, and to record the speed of their responses.
“In theory, if this is safe, and if we continue to prove that glyburide is efficacious in brain-injury prevention, every soldier could be taking this pill while in warfare, and therefore wouldn’t succumb to the secondary affects of brain injury,” Bochicchio says.“It could revolutionize how we treat and protect soldiers.”
Recruiting for the study began in December 2012.
Of his research at Barnes-Jewish Hospital, Bochicchio says, “I want EMTs in the area to know that we are doing things in the lab, as well as in our practice, to improve trauma care locally and nationally.”